
Supercapacitors
- Posted by doEEEt Media Group
- On December 14, 2022
- 0
Supercapacitors (or ultracapacitors) are one of the most progressing capacitor technologies in recent years, offering very high DC capacitance and high energy densities. It is proven its reliable and design flexibility to provide a wide range of energy storage solutions from small wearables, industrial applications, and automotive to large energy power network backup systems.
Sometimes all supercapacitors are miscalled as EDLC (Electric Double Layer Capacitors). However, EDLC is a subset of the supercapacitor family.
Supercapacitor’s features sit between capacitors and batteries, with a healthy cell-rated voltage between 1 and 3.8V. Since their introduction, supercapacitors have proved to be very reliable; with continuous long life operation and practically no charge/discharge cycle wear out.
Introduction and Basic Function
Supercapacitors are used as DC energy storage media, short high-power charge storage (automotive start-stop systems), backup for semiconductor memories and microprocessors, etc. New designs in larger modules have opened up space for several power applications that concur with rechargeable batteries.
There is no fixed dielectric material, and the charge is accumulated in the interface between active electrodes and the electrolyte. There are two basic mechanisms of charge storage:
- Electrostatic Charge Storage
- Pseudocapacitance Electrochemical Charge Storage
Electrostatic storage is based on the charge accumulation in charge traps within so-called Helmholtz layers (inner and outer) – see Figure 1. Pseudocapacitance storage is an electrochemical process where adsorbed ions accumulate charge. – see Figure 2.
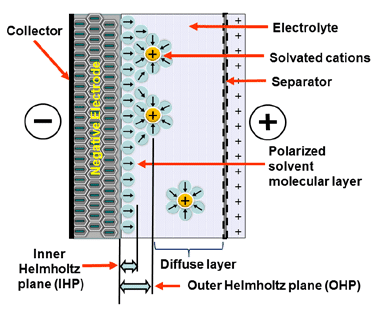
Figure 1. Electrostatic storage charge mechanism of supercapacitors
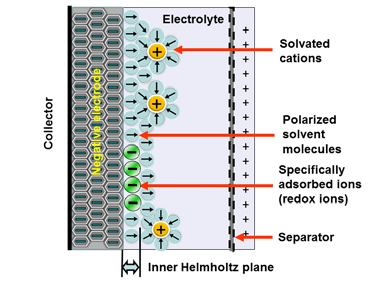
Figure 2. Electrochemical storage (Pseudocapacitance) charge mechanism in supercapacitors.
The mix of both charge storage mechanisms is present in real supercapacitors. The dominant charge mechanism that is present in supercapacitors defines three categories as shown in Figure 3.
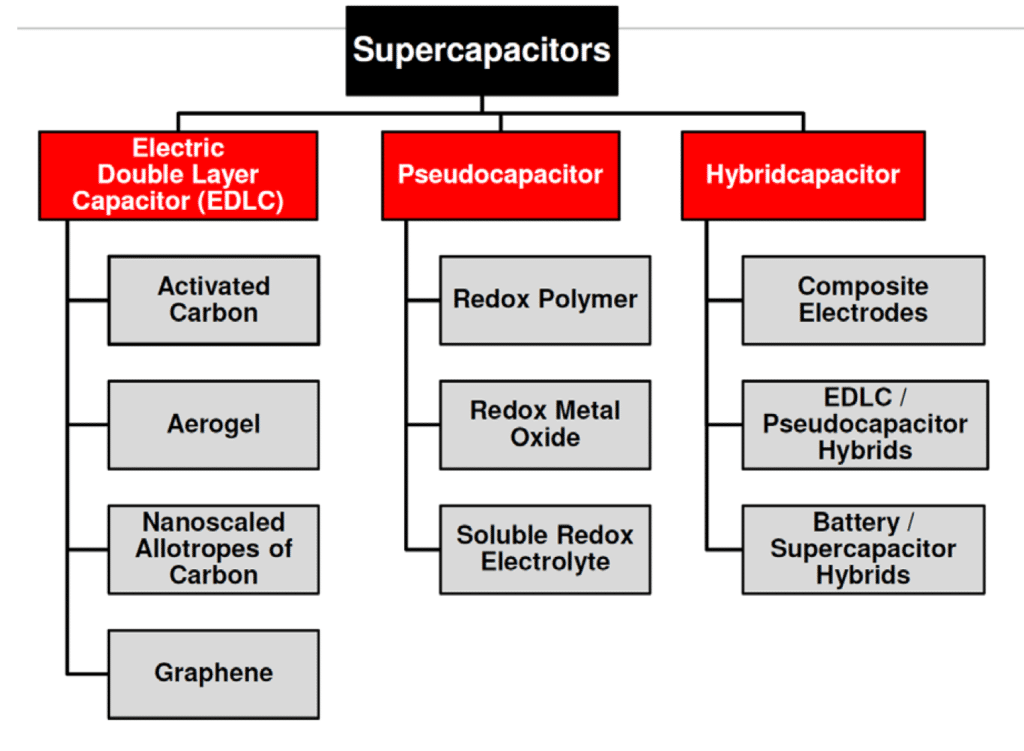
Figure 3. Supercapacitor types
EDLC capacitors use high-surface synthesized electrodes based on activated carbon, carbon nano-tubes, or graphene. Alternatively, the electrodes can be made from cheap “bio-waste” monolithic material with a natural hierarchy of pore sizes, such as coconuts, melon rinds, wood, fish scales, etc.
EDLC capacitors with symmetrical electrodes are non-polarized but are, in practice, supplied with a polarity marking that should be followed. One reason is that the positive electrode (+) may be processed differently from the negative one (-).
The electrochemical storage – pseudocapacitance – is not related to any electrochemical reaction – in difference to batteries. The charge can be stored by mechanisms such as redox-pseudocapacitance or redox-intercalation – see Figure 4. below.
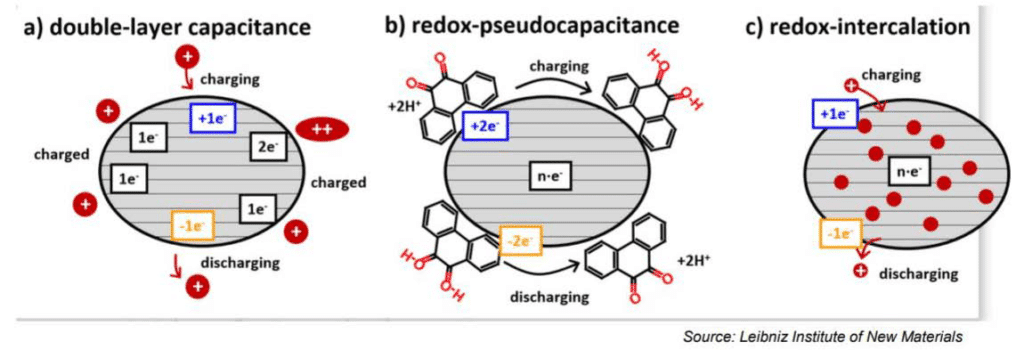
Figure 4. Supercapacitor storage mechanisms
It is also possible to combine hybrid designs with other electrode technology, such as
- capacitor hybrid: wet tantalum hybrid capacitor – one electrode tantalum anode and second electrode supercapacitors
- battery hybrid: supercapacitor one electrode and second battery electrode
Construction
Electrodes
Supercapacitor construction is explained on EDLC symmetrical structure. Nevertheless, the basic design concept is also valid for pseudocapacitors that target boosting electrochemical storage using different materials, processes, and electrolytes.
When we apply a voltage over the capacitor, existing ions in the electrolyte go through the membrane to their respective electrode, i.e., to the surface of the activated carbon that, via the electrolyte, is connected to the current supply electrodes. The ions are captured on the activated carbon surface, which attracts reverse charges inside the carbon (Figures 5. and 6.). We thus have a double layer of charges. Hence the name double layer capacitor. A schematic taken from a modern construction is shown in Figure 6. The original designs from the 1970s used a membrane and conductive rubber as a current collector, as shown in Figure 5. Modern constructions replace the membrane with a separating porous foil and the conductive rubber with a current collector. Usually an aluminum foil – see Figure 6. The structure of the wound – the stacked type using aluminum foil as a current collector, is shown in Figure 7.
Figure 5. illustrates how the activated carbon and the ions in the electrolyte work together.
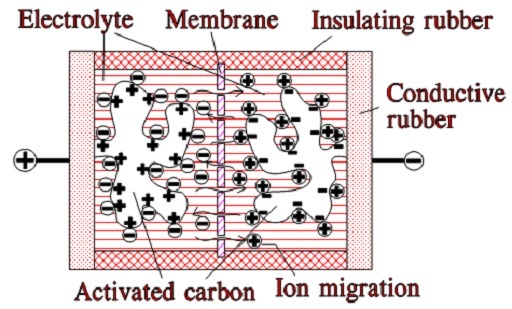
Figure 5. Function of EDLC supercapacitor (older construction)
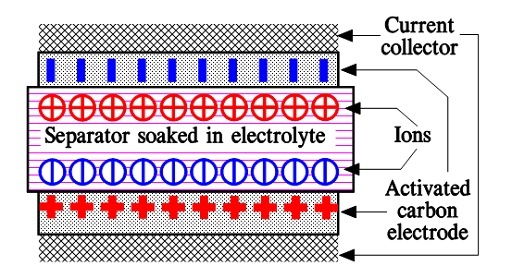
Figure 6. Schematic of EDLC supercapacitor
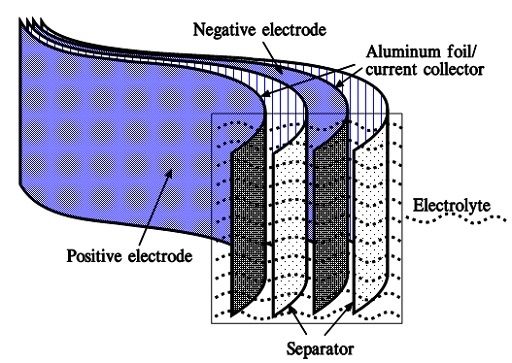
Figure 7. Wound EDLC supercapacitor structure with aluminum foil current collector
Because the distance between the charges is small – ion diameters –and furthermore, because the total carbon surface is enormous, the charge quantity will be extremely large. The capacitance range amounts to the magnitude of several thousand farads.
The continuous development to enlarge surface area has resulted in sophisticated, active electrode systems based on active carbon layer (carbon fibers), carbon nano-tubes (CNT), or the latest design with graphene.
Electrolyte
Applied voltage, efficiency, and power handling also depend on a selection of electrolytes. Electrolytes provide a media that supports the creation of charge on the interface with electrodes, enables its mobility, or provides adsorbed ions as charge carriers (pseudocapacitance). Electrolyte matching with the electrode system is thus essential to achieve maximum energy and power density and define cell voltage.
There are currently three types of electrolytes:
- aqueous based
- organic-based (liquid or solid/gel)
- ionic liquids
Aqueous electrolytes provide good conductivity at no toxicity. However, the maximum voltage reaches 1.2 V.
Organic electrolytes can have a maximum of ~3 V and provide a better temperature range. Nevertheless, they can be limited by flammability or toxicity. Solid organic electrolytes usually contain conductive polymers with low ESR values and corresponding power pulse capabilities.
The latest electrolyte development steps. Ionic liquids are salts in liquid form rich in ions and short-lived ion pairs. This electrolyte increased maximum voltage to ~3.7 V at no issue with flammability or toxicity – see spider chart benchmarking electrolyte types in Figure 8.
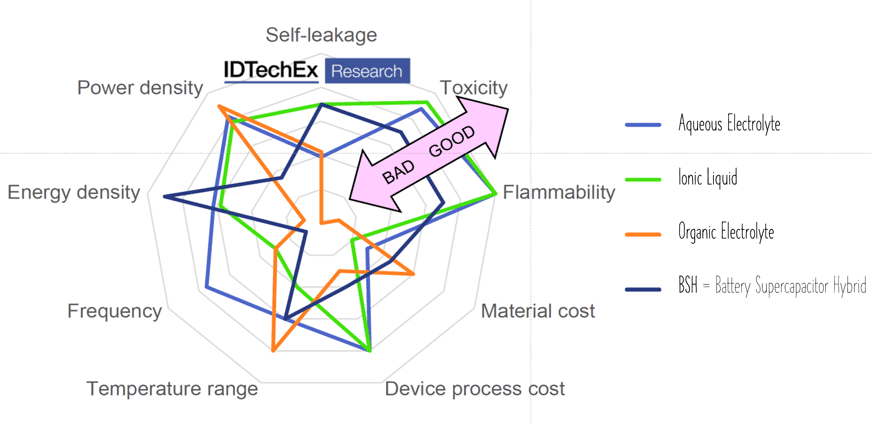
Figure 8. Supercapacitor electrolyte types comparison; source: IDTechEx, used under permission
If the voltage exceeds the maximum cell voltage, the electrolyte decomposes to form H2 and O2. The surge voltage is specified below that voltage, and with some margins to the surge voltage, we find the rated cell voltage.
ESR Resistance
The active electrode (carbon) particles in the dispersion variant connect via the electrolyte and adjacent particles with the current supply electrodes. Some particles are situated close to its electrode and have a comparatively small contact resistance; others have long contact chains and manifold larger connection resistances. Figure 9 shows three particles with the series resistances R1, R2, and Rx, where R1 < R2 << Rx. The figure also shows the membrane resistance Rm in the electrolyte. The carbon particles are not spheres but have a surface with hollows and channels, just like the etched surface of aluminum foil, but even more, enlarged. See the schematic enlargement in the figure.
The electrolyte resistance from the inlet to the bottom of a channel will be considerable. The charges in the channel get a varying contribution of series resistances, depending on location in the channel, a contribution that shall be added to those resistances R1, R2…., intimated in the entire view.
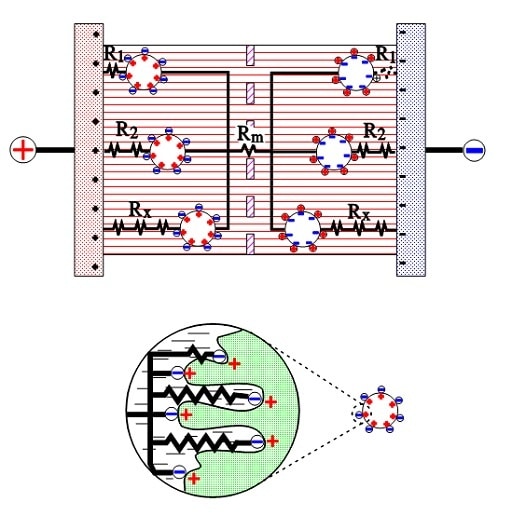
Figure 9. Electrolyte and contact resistances in the double-layer supercapacitor.
This results in many elementary capacitances mutually connected in parallel in a complicated resistor network whose part resistances differ between themselves with several powers of ten. The time constants of the respective elementary capacitances vary from fractions of a second to hundreds of hours. The resistance network can be summarized as an ESR that varies with capacitance, type, and manufacture from milliohms to several hundred ohms at RT. What contributes to the lower ESR values of modern ultracapacitors is the more conductive organic electrolytes or ionic liquids and improvements in the contact medium between the active electrode particles and current collectors.
Because the ESR in many backup capacitors is large compared to aluminum electrolytic, it limits the ripple current use. The usual limit for the heat release is set to +2 °C.
We don’t have any real dielectric, only a face boundary between electrode and electrolyte of 2 to 5 nm that prevents separate charges from passing.
Series connection
Energy increase with voltage squared; thus, modern high-power electronics require work in 16, 25, 35, 50, and 110-volt ranges, which requires multiple cells linking (2 to 4 V). The automotive market push towards 48-volt subsystems.
That raises concern about the reliability of units containing multiple cells linked together. If we in electronic designs, want to connect discrete capacitors in series to meet higher working voltages, we should use the same type of elements.
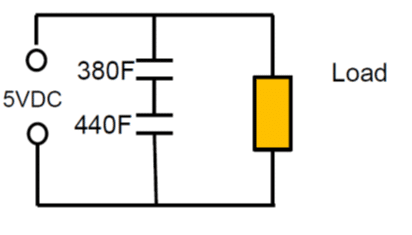
Figure 10. supercapacitor series connection
As an example: Series connection of 2pcs 400F 2.5V cells with +10/-5% cap tolerance in worst case scenario will end up with 380F and 440F caps on the board. The voltage of the individual cells will split accordingly to: 2.68 V: 2.32V, which exceeds the rated voltage of the first capacitor.
As noted from electrolyte decomposition, the rated cell voltage of supercapacitors (or surge voltage, if specified) must not be exceeded. Exceeding the maximum cell voltage considerably lower the lifetime of supercapacitors; thus use of balancing circuits is strongly recommended when connecting unit cells in series to attain higher-rated voltages.
Picked from a manufacturer datasheet: The rule of thumb by EDLC supercapacitor lifetime prediction is:
- With every 0.2 voltage decrease, the cell lifetime increases about 2x in the specified voltage range
- With every 0.1 voltage increase over the spec V, the cell lifetime gets half
see figure11. Comparison of balancing methods and their impact on lifetime and efficiency.
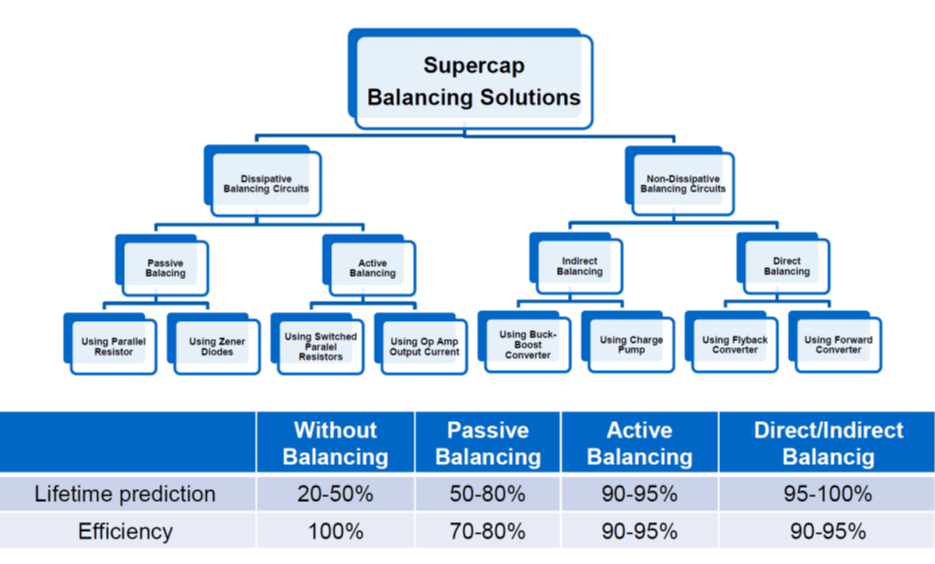
Figure 11. balancing method impact to lifetime and efficiency of EDLC supercapacitors; Source: Eaton
If we don’t use external voltage dividers, it is recommended as a precaution that the applied total voltage divided by the number of linked cells does not exceed 85% of the rated cell voltage.
Note: Low voltage (~1.8V) aqueous electrolyte type may not require balancing on higher voltage modules as the variability of applied voltage on the high number of cells/layer may not be critical.
The Time Constant, t
The time constant of capacitors is the time needed to discharge a loaded capacitor through the insulation resistance (IR) to 1/e (~37%) of the initial voltage. This constant, denominated t(tau), is the product of IR and C (capacitance), usually abbreviated RC. Supercapacitors don’t have any dielectric even if the actual leakage current represents a kind of “internal DC resistance.” What is of importance to supercapacitors is their ability to absorb and release electric charges. This depends on their ESR. The more conductive the electrolyte and the better construction that facilitates the current flow, the lower the ESR and, consequently, the faster the charge and discharge time.
Some supercapacitors have many time constants, while especially power-burst designs are characterized by tiny ones, ranging from milliseconds to minutes.
Supercapacitors’ Energy Density (Wh/kg) and Power Density (W/kg)
Compared to conventional electrolytic capacitors, Electrochemical capacitors have approximately 10-100 times higher energy density (also called specific energy) expressed in Wh/kg. If we compare it with traditional electrostatic capacitors, the particular energy is approximately 100 times higher. Despite supercapacitors being subjected to fast developments, batteries may typically have 10 to 100 times as much energy density (as battery development is also significantly moving forward).
Comparisons on power density show another picture. Electrochemical capacitors having considerable ESR values and time constants of 1 to 100 seconds equal batteries concerning power density. In recent years marketed power supercapacitors that can deliver ten times the power of conventional batteries or, with the latest graphene electrode design, can get very close to electrolytic capacitors. Their time constants range from below 0.1 seconds to 30 seconds.
Applications
As mentioned in the introduction, supercapacitors seem very reliable, claiming a service life exceeding ten years. Together with capabilities of charge/discharge cycles ranging to millions, they offer maintenance-free products of high interest, especially when combined with rechargeable batteries. They have a high power density, and their capacitance ranges from several farads to several thousand farads, making them perfect for high power-burst applications. Nevertheless, watch for low-end “no-name” supercapacitors, as there are also “cheap” technologies on the market with significantly worse capacitance drop versus cycles/lifetime or operation at higher temperatures.
The high power-burst applications can be divided into two categories. The first one is small-cell designs. They belong mainly to applications like digital cameras, wireless PCs, and the like, where they are used to load-level pulses from such energy sources as batteries and fuel cells.
The second category is large-cell types. For example, they will be found in automotive applications and as UPS (uninterrupted power supplies) in industrial applications. Whether small-cell or large-cell supercapacitors are utilized in power-burst applications, they will relieve the battery of pulsed power functions. That means the possibility to utilize smaller power batteries.
In electrical vehicles (EV) and hybrid electric vehicles (HEV), a combination of batteries and supercapacitors provides high immediate power and a longer energy supply. The supercapacitor is also a popular solution for start-stop systems integrated into vehicle energy recuperation energy management systems to lower the emission and consumption of combustion engine vehicles (CE) and HEV. As already mentioned, these supercapacitors can deliver more than ten times more power than batteries. Especially at lower temperatures, where the power supply from batteries might be insufficient,, supercapacitors can be the solution. Some of them can operate successfully down to –40°C. Batteries have minimal power in cold, and the power supply for starting may be pretty insufficient. They can, on the other hand, trickle-charge the supercapacitor that, in turn, has the power necessary for creating.
Many of these supercapacitors can be discharged down to 0 V. On the other hand, one major limitation of supercapacitors blocking the complete replacement of batteries in combustion engine vehicles is their higher self-discharge/DCL and its significant increase at higher temperatures – see further below and illustration in Figure 21.
Charge and Leakage currents
In aqueous electrolyte constructions, as well as in some organic electrolyte types, we could, concerning time, distinguish three different types of current components:
- a charging current, Ich,
- an absorption current, Labs,
- a leakage current, DCL.
The charge current charges the different capacitor elements. A large part of this “operating charge” can occur within fractions of a second but the exchange increases if we extend the time to minutes or even more. Even if ESR limits the surge current at rapid charges/ discharges, a current limiting resistance of the same magnitude as the ESR is recommended.
As was seen in the previous section, there is an extensive distribution of time constants for the different capacitor elements. The most inert ones never will be charged within a reasonable time. Once they are set, they won’t participate in discharges under a specific practice time. They have been absorbed. The so-called absorption current characterizes the absorption process during charging.
After a very long charging time, we reach a limit for all charging currents. There is a current left, the actual leakage current. In reality, it is independent of time since it exists all the time. Figures 12. and 13. show examples of the three current components and their time dependence.
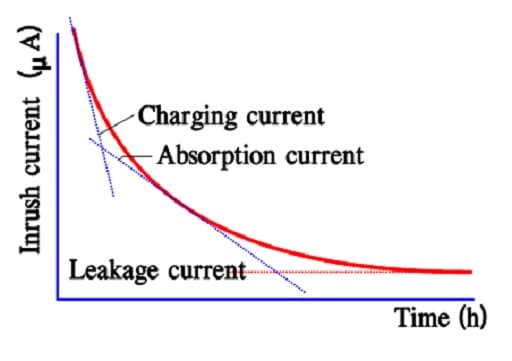
Figure 12. The three components of the inrush current in a double-layer supercapacitor.
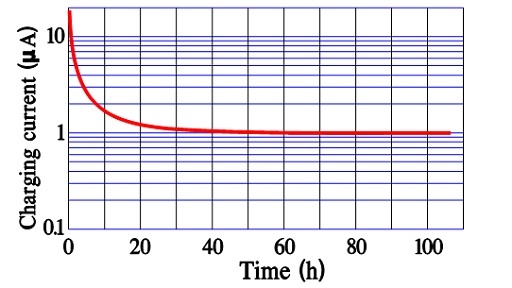
Figure 13. An example of Inrush current versus time in an older double-layer supercapacitor.
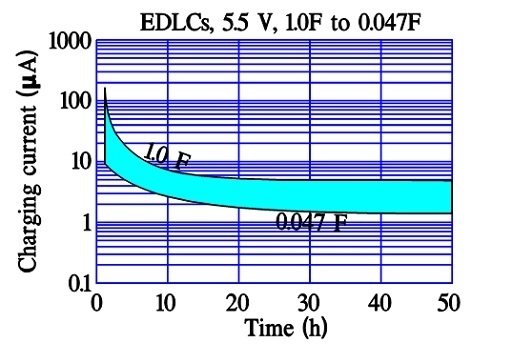
Figure 14. Examples of inrush currents versus time in double-layer supercapacitors.
In modern power supercapacitors, the charge/discharge times are so small that no significant difference exists between the first two current components discussed above.
Measurements
Leakage current
The leakage current, DCL, is sometimes called bias current and is specified after 10, 30, or 60 minutes of applied rated voltage, as seen in Figures 12. and 13. these times are too short for an accurate DCL determination. For reciprocal comparisons, however, they serve their purposes.
Remarkably, some manufacturers of power supercapacitors (where the charge/discharge time is specified from 0.3 to 30 seconds) specify DCL measurements after 12 or 72 hours of applied rated voltage.
Capacitance
Despite decreasing ESR values in modern designs, AC capacitance measurements are considered inaccurate. Instead, a discharge with constant current is resorted to (Figure 15.).
The capacitance determination is based on the fundamental formula Q = C x V. But, at constant current, Q = I x t.
According to the figure: C x (V1-V2) = I x (t2-t1).
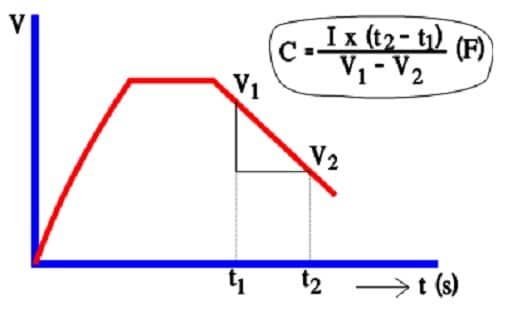
Figure 15. Principle of capacitance determination of a double layer supercapacitor.
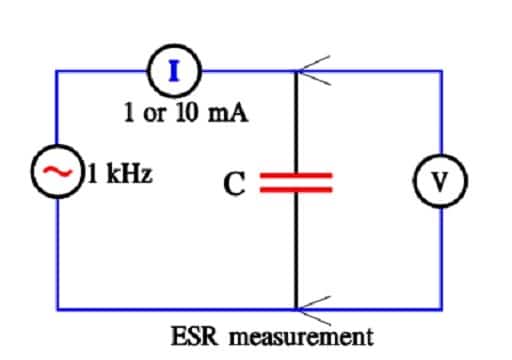
Figure 16. Determination of supercapacitor ESR.
ESR
ESR is measured at 1 kHz and a constant current of 1 or 10 mA.
Life calculations
Supercapacitor manufacturers mainly specify estimated life versus voltage and temperature. Nevertheless, it can be specific to the supercapacitor technology, so it is recommended to check the manufacturer datasheets.
Roughly the life will be reduced by more than two times (more accurately 2.23 times) for every 10 degrees C increase. If we use the formula [1], it should be written as:
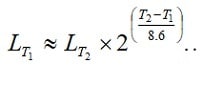
[1]
Life dependence of working voltage can be expressed with the formula [2]. Because we deal with electrolytes the exponent is significantly higher than that of electrostatics, i. e., n » 17, which gives us the following equation:
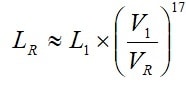
[2]
According to the manufacturer’s graphs (and the formula above), there will be an approximate halving of life for every 4.4 % voltage increase above VR and a corresponding life increase for derating with 4.4 %.
Environmental operation risks
Avoid the use of non-hermetic types at:
- environments with a high RH
- corrosive gases
- cleaning agents with Freon TMC or Trichloro-ethylene.
Characteristics
Discharging
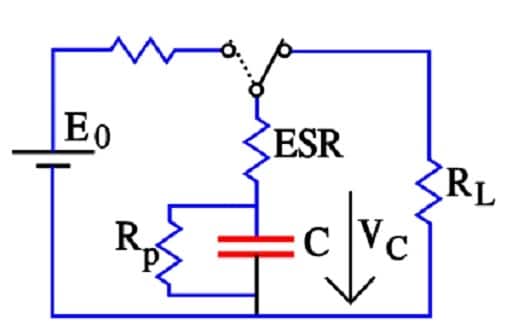
Figure 17. Discharge of a supercapacitor with an inductive load RL
At a discharge of the capacitor, the voltage follows the equation
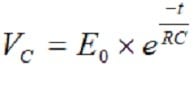
[3]
, where:
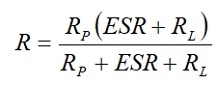
[4]
But at 1 to 100 mA load RL,>> ESR and Rp >> RL. Then R ≈ RL and the discharge time t = – CRL ln(VC/E0). Semiconductor memories, on the other hand, will draw less than 10 *A. In these cases, RL >> ESR and
R ≈ RpRL/(Rp+RL).
The following diagram in Figure 18. is taken from either of the established manufacturers and may differ between the manufacturers.
Discharge current versus backup time
Manufacturers of supercapacitors used for backup purposes specify voltage versus backup time/holding time for different discharge currents. The time is related to discharge from rated voltage down to 2 V (where its backup function ends).
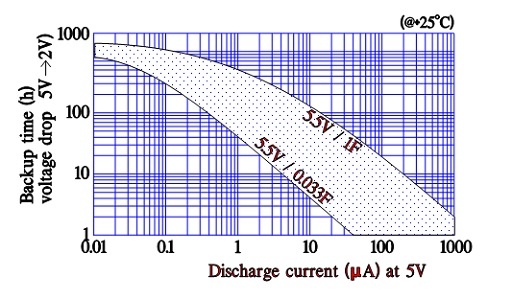
Figure 18. Supercapacitor backup time versus discharge current and capacitance.
Discharge conditions
The following figure shows a diagram of constant-current discharge curves for an EDLC supercapacitor with an ESR of a maximum of 120 ohms at 1 kHz. Note the sudden voltage drop of 0.5 V in the one mA curve. It is related to the internal DC resistance. At 0.1 mA, it is not visible.
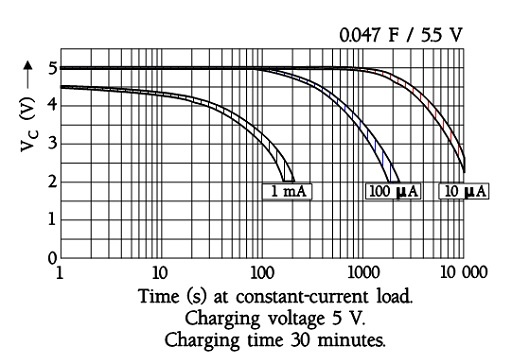
Figure 19. Example of discharge diagrams for a button cell EDLC supercapacitors.
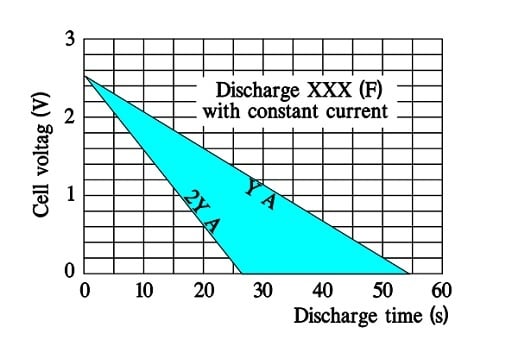
Figure 20. A typical discharge diagram for a large supercapacitor.
Discharge curves for ultracapacitors with small ESRs exhibit straight discharge curves. Fictitious curves for a large supercapacitor capable of a discharge to 0 V may have the following appearance where the discharge currents are called Y and 2Y (A).
Note that doubling the discharge current means halving the discharge time.
Self-Discharge
The internal leakage of electric charges will gradually decrease the initially applied voltage. The following two figures illustrate the phenomenon.
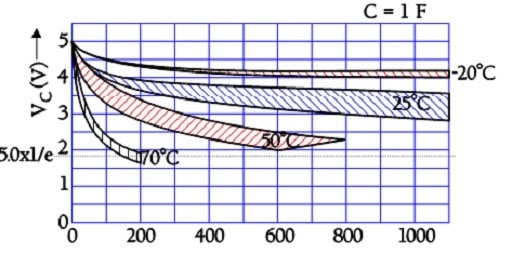
Figure 21. Examples of a supercapacitor temperature-dependent voltage drop at open circuit conditions and an internal leakage through the insulation resistance (self-discharge).
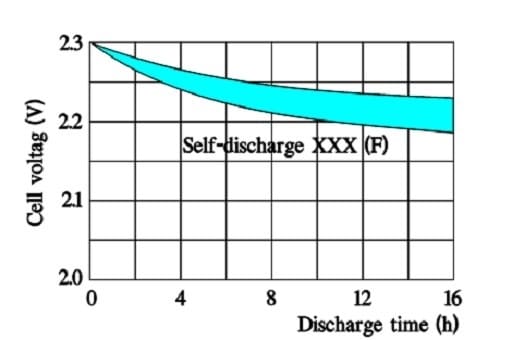
Figure 22. Typical appearance of the self-discharge curve for a larger supercapacitor with VR = 2.3 V.
The above figure shows that the time constant – i.e., the time it takes for the voltage to drop to 1/e of the initial voltage – is considerable, even at +70 °C. Larger supercapacitors characterized by lower ESR values may have the following self-discharge curve. Always consult the manufacturer for accurate specifications.
Temperature dependence
The typical capacitance dependence of temperature may look like the following curves in Figure 23.
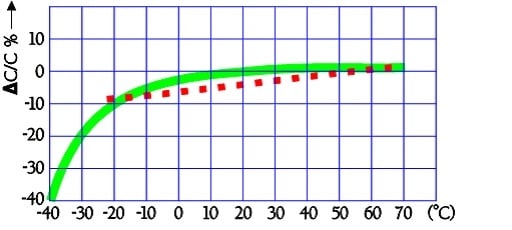
Figure 23. Typical examples of capacitance versus temperature. The bald-line curve represents a high-ESR carbon double-layer capacitor, and the dotted line is a solid electrolyte EDLC.
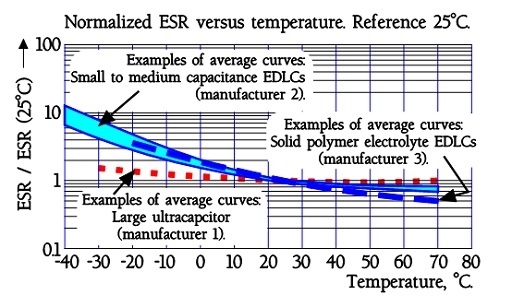
Figure 24. Normalized EDLC supercapacitor ESR versus temperature.
The quotient ESR-25°C / ESR+20°C will be ≤ 5. Larger modules are specified ESR-25°C / ESR+20°C ≤ 10.
The following diagram shows curves for the temperature dependence of the leakage current in older aqueous electrolyte capacitors. Because the actual double-layer construction is also valid in newer EDLCs, we have reason to believe that the temperature dependence is still present even if the magnitude may differ.
The leakage current examples in Figure 25. are based on readings after 60 minutes of electrification with 5.5 V dc. Charging for 10 or 30 minutes will, of course, give other values.
The quotient DCL70°C /DCL 20°C will be ≤ 4.
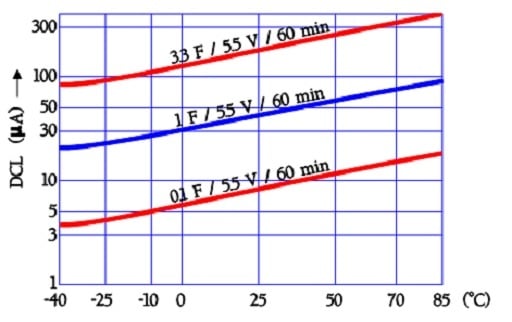
Figure 25. Examples of EDLC supercapacitor leakage current versus temperature.
Source: Epci Blog
- Space-Grade components available for immediate delivery - April 10, 2025
- Exclusive stock on doEEEt: How to access and request - April 10, 2025
- Managing EEE components for LEO and lower cost space missions - December 17, 2024

0 comments on Supercapacitors