Energy storage future enabled by nanomaterials
- Posted by doEEEt Media Group
- On May 28, 2020
- 0
Science Mag published a insight into use of nanomaterials for energy storage devices. Despite the key focus is towards batteries, indeed the observations and notes are valid for supercapacitors such as lithium-ion supercapacitors.
Thinking small to store more
From mobile devices to the power grid, the needs for high-energy-density or high-power density energy storage materials continue to grow. Materials that have at least one dimension on the nanometer scale offer opportunities for enhanced energy storage, although there are also challenges relating to, for example, stability and manufacturing. In this context, Pomerantseva et al. review fundamental processes of charge storage that apply specifically to nanostructured materials and briefly explore potential manufacturing processes. The authors also consider some of the scepticism, such as that found in the battery community, to the use of these materials.
Background
Nanomaterials offer greatly improved ionic transport and electronic conductivity compared with conventional battery and supercapacitor materials. They also enable the occupation of all intercalation sites available in the particle volume, leading to high specific capacities and fast ion diffusion. These features make nanomaterial-based electrodes able to tolerate high currents, offering a promising solution for high-energy and high-power energy storage. However, there are still many challenges associated with their use in energy storage technology and, with the exception of multiwall carbon-nanotube additives and carbon coatings on silicon particles in lithium-ion battery electrodes, the use of nanomaterials in commercial devices is very limited. After decades of development, a library of nanomaterials with versatile chemical compositions and shapes exists, ranging from oxides, chalcogenides, and carbides to carbon and elements forming alloys with lithium. This library includes various particle morphologies, such as zero-dimensional (0D) nanoparticles and quantum dots; 1D nanowires, nanotubes, and nanobelts; 2D nanoflakes and nanosheets; and 3D porous nanonetworks. Combined with lithium and beyond lithium ions, these chemically diverse nanoscale building blocks are available for creating energy storage solutions such as wearable and structural energy storage technology, which are not achievable with conventional materials.
Advances
The success of nanomaterials in energy storage applications has manifold aspects. Nanostructuring is becoming key in controlling the electrochemical performance and exploiting various charge storage mechanisms, such as surface-based ion adsorption, pseudocapacitance, and diffusion-limited intercalation processes. The development of new high-performance materials, such as redox-active transition metal carbides (MXenes) with conductivity exceeding that of carbons and other conventional electrode materials by at least an order of magnitude, open the door to the design of current collector–free and high-power next-generation energy storage devices. The combination of nanomaterials in hybrid architectures, such as carbon-silicon and carbon-sulfur, together with the development of versatile methods of nanostructuring, overcome challenges related to large volume change typical for alloying and conversion materials. These examples indicate that nanostructured materials and nanoarchitectured electrodes can provide solutions for designing and realizing high-energy, high-power, and long-lasting energy storage devices.
Outlook
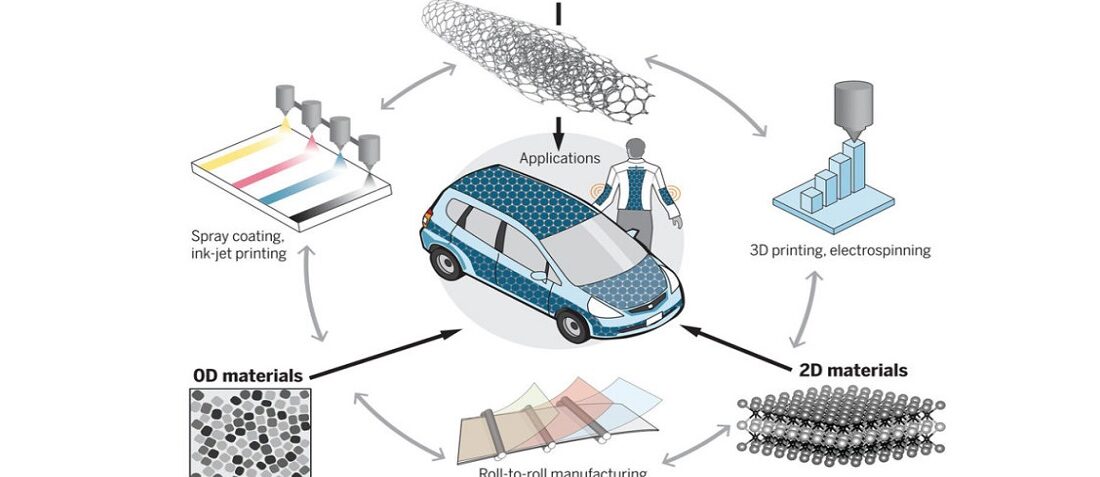
The limitations of nanomaterials in energy storage devices are related to their high surface area—which causes parasitic reactions with the electrolyte, especially during the first cycle, known as the first cycle irreversibility—as well as their agglomeration. Therefore, future strategies aim to develop smart assembly of nanomaterials into architectures with controlled geometry. Moreover, combining nanomaterials with complementary functionalities, such as high electronic conductivity of graphene or MXenes with high operating voltage and high redox activity of oxides, is necessary. Building sophisticated electrode architectures requires innovative manufacturing approaches, such as printing, knitting, spray deposition, and so on. Already-developed techniques such as 3D printing, roll-to-roll manufacturing, self-assembly from solutions, atomic layer deposition, and other advanced techniques should be used to manufacture devices from nanomaterials that cannot be made by conventional slurry-based methods. Such manufacturing approaches can also enable long-sought flexible, stretchable, wearable, and structural energy storage and harvesting solutions for Internet of Things and other disruptive technologies.
Featured image: Nanomaterials for energy storage applications. Image credit: ScienceMag
The high surface-to-volume ratio and short diffusion pathways typical of nanomaterials provide a solution for simultaneously achieving high energy and power density. Furthermore, the compatibility of nanomaterials with advanced manufacturing techniques—such as printing, spray coating, roll-to-roll assembly, and so on—allows for the design and realization of wearable, flexible, and foldable energy storage devices.
Lithium-ion batteries, which power portable electronics, electric vehicles, and stationary storage, have been recognized with the 2019 Nobel Prize in chemistry. The development of nanomaterials and their related processing into electrodes and devices can improve the performance and/or development of the existing energy storage systems. We provide a perspective on recent progress in the application of nanomaterials in energy storage devices, such as supercapacitors and batteries. The versatility of nanomaterials can lead to power sources for portable, flexible, foldable, and distributable electronics; electric transportation; and grid-scale storage, as well as integration in living environments and biomedical systems. To overcome limitations of nanomaterials related to high reactivity and chemical instability caused by their high surface area, nanoparticles with different functionalities should be combined in smart architectures on nano- and microscales. The integration of nanomaterials into functional architectures and devices requires the development of advanced manufacturing approaches. We discuss successful strategies and outline a roadmap for the exploitation of nanomaterials for enabling future energy storage applications, such as powering distributed sensor networks and flexible and wearable electronics.
Advances and phenomena enabled by nanomaterials in energy storage
Nanostructuring often enables the use of conventional materials that cannot be used in the microcrystalline state as either cathodes or anodes. Classical examples are alloying anodes—such as silicon, germanium, or tin—that experience large structure and volume changes during cycling. Bulk silicon, which has a theoretical capacity of up to 3579 mA·hour g−1, considering Li15Si4 formation, cannot work as stand-alone anode in a Li-ion battery. The life cycle of silicon-based anodes is limited by the pulverization of the active material, which is determined by the volume swelling of silicon upon lithiation (up to 400 volume %) and subsequent shrinkage upon delithiation. However, reducing the particle size below ~150 nm limits the electrode cracking upon the insertion of Li+ ions, which mitigates the anode mechanical failure. There have been designs proposed to overcome the issues of large volume expansion and mechanical failure, including the use of nanowires, nanotubes, graphene flakes, hollow spheres, and core-shell and yolk-shell structures. To build a stable SEI for nanomaterials with the large volume change, the concept of nanoscale double-walled hollowed structures was demonstrated. In this structure, the outer wall confines the expansion of the inner wall toward the hollow space inside and therefore generates a static outer surface for stable SEI formation.
Nanomaterials with fast ion and electron transport
Low-dimensional materials can combine high electronic and ionic conductivities by using a mechanism that is usually referred to as pseudocapacitive or surface redox energy storage. Transition-metal atoms on the surface of MXenes can participate in redox reactions with fully electrochemically reversible redox wave in cyclic voltammetry curves overlaid on the large rectangular area corresponding to the double-layer capacitive charge storage mechanism. The example of MXenes has shown that both double-layer and redox capacitance can be used at very high current rates, with just ~20% electrochemical performance loss when going from 10 to 100,000 mV s−1 cycling. This rate would be impossible for conventional redox electrodes, which have low conductivity and a diffusion-limited charge-storage mechanism. MXenes have shown a charging time in the 1- to the 10-ms range. At the same time, the capacitance of MXenes (up to 500 F g−1 and 1500 F cm−3 in thin films in acidic electrolyte) exceeds the capacitance of double-layer capacitor materials, such as carbons, which have 100 to 200 F g−1 or F cm−3, while volumetrically rivaling that of ruthenium oxide thin films (1500 F cm−3). Thick electrodes can also work well, if restacking of the 2D sheets is prevented. In fact, vertical alignment of 2D sheets, achieved by exploiting their liquid crystalline behavior or through templating, would allow the development of MXene electrodes with tens of milligrams per square centimeter. In this context, thickness-independent (up to 200 μm) capacitance of vertically aligned MXene flakes has been demonstrated.
In many cases, however, it is necessary to combine different materials to achieve fast transport of both electrons and ions. A good example is a design and realization of hybrid structures, which have been reported for numerous oxides (Nb2O5, TiO2, MoO3, etc.) on a variety of carbon supports, such as nanotubes, graphene-based materials, and porous carbons. The carbon affects the electronic properties of both materials because it not only acts as a channel for electrons but also forms a heterojunction between the oxide and carbon surface. As a result, a higher capacity (~1000 mA·hour g−1) has been achieved for a graphene–iron oxide electrode compared with both only oxide (~600 mA·hour g−1), which cannot operate at high rates, and only carbon material (~400 mA·hour g−1). When combined, these materials can operate at current densities exceeding several amperes per gram. Moreover, with the correct design of the electrode architecture, a very high rate performance can also be achieved, as demonstrated for Nb2O5 supported on graphene or MXene. Building such composite architectures can also allow the use of conversion electrode materials, such as FeF3, CuCl2, or S undergoing phase transformations.
Another way to enable fast transport of electrons and ions is through the creation of 2D heterostructures, which allow the combination of highly conducting and high–energy density 2D materials. Because at least one material in the hybrid structure should have good electronic conductivity, graphene has been the primary material of choice. This approach is rather universal, with a very large number of metallically conductive and redox-active materials available. It has been implemented in several different systems for applications ranging from pseudocapacitors to Li-ion and Li-S batteries. As of now, the governing assembly principles of integrating dissimilar nanomaterials into desired architectures are poorly understood. Moreover, it is not yet known how the transport of electrons and ions occurs between dissimilar 2D sheets and through the separating electrolyte or confined fluid. What is the optimal spacing between the sheets? Is the physical contact between the particles an essential requirement for electron transport or can the hopping or tunnelling serve as the dominant transport mechanism? Machine learning should allow for optimization of those systems and for understanding the guiding principles for the selection of the optimum combinations of 2D materials to achieve the best electrochemical performance.
The original article: “Energy storage: The future enabled by nanomaterials” by Ekaterina Pomerantseva et col. appeared at ScienceMag. Vol. 366, Issue 6468, eaan8285. DOI: 10.1126/science.aan8285
Source: ScienceMag
- Miniature RF Connectors for high-performance testing - April 24, 2025
- Space-Grade components available for immediate delivery - April 10, 2025
- Managing EEE components for LEO and lower cost space missions - December 17, 2024
0 comments on Energy storage future enabled by nanomaterials